Hydrogen: Why We Need It - and Why It Needs to Change

Hydrogen has gone from niche topic to major headline over the last few years. Some hail it as the carbon-free fuel of the future; others dismiss it as inefficient and overhyped. As an engineer, I’m not interested in emotional bias. I want to understand why we need it at all, whether it really needs to go green - and if so, how we might get there.
Simple fact: hydrogen already plays a massive role in our global economy - it’s just hidden in products we use everyday.
According to the International Energy Agency, 97 million tonnes of hydrogen were produced globally in 2023. That sounds like a lot - and it is. To put it into perspective, that’s almost half the amount of natural gas used in the European Union that same year. But where does all that hydrogen go?
Hydrogen quietly powers the modern world
We don’t see hydrogen in our everyday lives, but it's embedded in some of the most impactful industrial processes on earth:
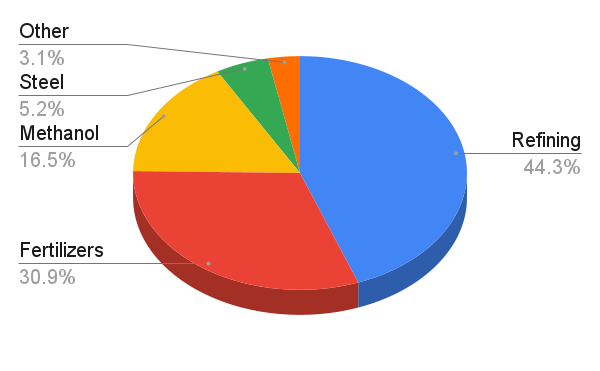
Crude Oil Refining – ~43 million tonnes

Do you remember when acid rain was a major environmental concern? I do - vivid images of dying forests and corroding buildings filled the encyclopedias I read as a child. But these days, acid rain is rarely mentioned. Why? Because of desulfurization - and hydrogen plays a key role in that.
The largest single use of hydrogen today is in crude oil refining, which consumes nearly half of all global hydrogen. It’s used to remove sulfur from crude oil during the production of low-sulfur diesel and to treat gasoline. In these processes, hydrogen reacts with sulfur compounds to form hydrogen sulfide (H₂S). This is then extracted and later converted into elemental sulfur. Interestingly, that is how the majority of the world's sulfur is produced (but that's a story for another time).
The goal isn’t sulfur production. It’s to prevent sulfur dioxide (SO₂) emissions - a major contributor to acid rain. Thanks to stricter regulations and hydrogen-based refining, SO₂ emissions from vehicles have dropped dramatically in Europe and North America and thus the threat of acid rains.
Fertilizer – ~30 million tonnes

The next largest hydrogen consumer - nearly a third of global demand - is the fertilizer industry.
It may sound mundane, but it changed the world: without modern nitrogen fertilizers, we couldn’t have grown enough food to support today’s global population.
About 210 million tonnes of fertilizer are produced each year. Over half of that is nitrogen-based - such as urea and ammonium nitrate - and requires ammonia as a feedstock. Ammonia, in turn, is produced by combining nitrogen (from air) with hydrogen under high pressure and temperature in the Haber-Bosch process - one of the most important chemical reactions in history. Without ammonia, and it turn hydrogen, modern agriculture as we know it wouldn't exist.
Methanol – ~16 million tonnes

Hydrogen is also used to produce methanol - historically known as wood alcohol. Industrially, methanol is made from syngas, a mixture of carbon monoxide (CO), CO₂, and hydrogen, typically produced via steam methane reforming (SMR) of natural gas.
This gas mixture is fed into a reactor at around 250 °C, where it passes over a copper-zinc oxide catalyst to form methanol. Since the hydrogen-to-carbon ratio in syngas isn’t always optimal, additional hydrogen is often added to balance the reaction and improve yield. Around 16 million tonnes of hydrogen are used annually for this purpose - making up more than half of global methanol production.
Methanol is widely used as a fuel or fuel additive, but more importantly, as a feedstock for chemicals like formaldehyde and acetic acid. These, in turn, are used to make glues, solvents, paints, and plastics like PET - found in everything from soda bottles to textiles. It's also one of the most affordable solvents, making it a key ingredient in the pharmaceutical industry.
Steel – ~5 million tonnes

An emerging but growing use of hydrogen is in the steel industry. About 5 million tonnes of hydrogen were used in 2023 to produce direct reduced iron (DRI) - a lower-emission alternative to traditional coal-based steelmaking.
In this process, hydrogen reacts with iron ore (iron oxide) at 800-1,200 °C, removing the oxygen and producing solid sponge iron and water vapor. The sponge iron is then melted in an electric arc furnace (EAF) to produce steel.
If powered by green electricity and green hydrogen, this method can dramatically cut CO₂ emissions. While still a small share of total steel output, hydrogen-based DRI is promising - particularly for high-grade steel in sectors like automotive, where recycled scrap metal may not be pure enough.
The CO₂ Cost of Hydrogen
So far, hydrogen sounds like an industrial workhorse. But there’s a catch: most of it is made using fossil fuels.
- Around 60% is produced from natural gas, primarily through SMR. In this process, methane reacts with steam at 800–900 °C to produce hydrogen and CO₂.
- Another 20% is made from coal, via coal gasification, which also releases large amounts of CO₂.
The rest comes from oil-based sources, electricity (1 Mt), or as industrial byproducts. And the carbon cost is enormous:
- SMR emits 10–12 tonnes of CO₂ per tonne of hydrogen.
- Coal gasification emits 22–26 tonnes of CO₂ per tonne.
Altogether, global hydrogen production results in ~920 million tonnes of CO₂ annually - about 2.5% of global CO₂ emissions. That’s comparable to the entire aviation industry.
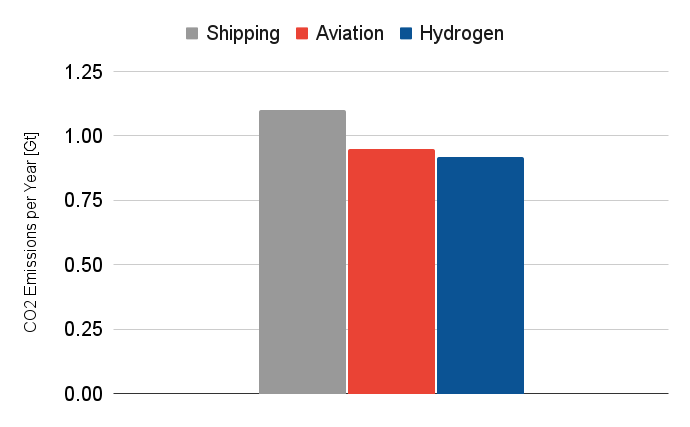
So even though hydrogen enables cleaner fuels, food production, and industrial materials, the way we produce it today comes with massive environmental downsides.
How do we decarbonize hydrogen - without losing the benefits it provides?
The Road to Clean Hydrogen
There are several options on the table for producing clean hydrogen. Some are already being tested at scale, others are still emerging. Each comes with its own trade-offs - and none is a silver bullet. But together, they help outline a potential roadmap toward cleaner hydrogen production.
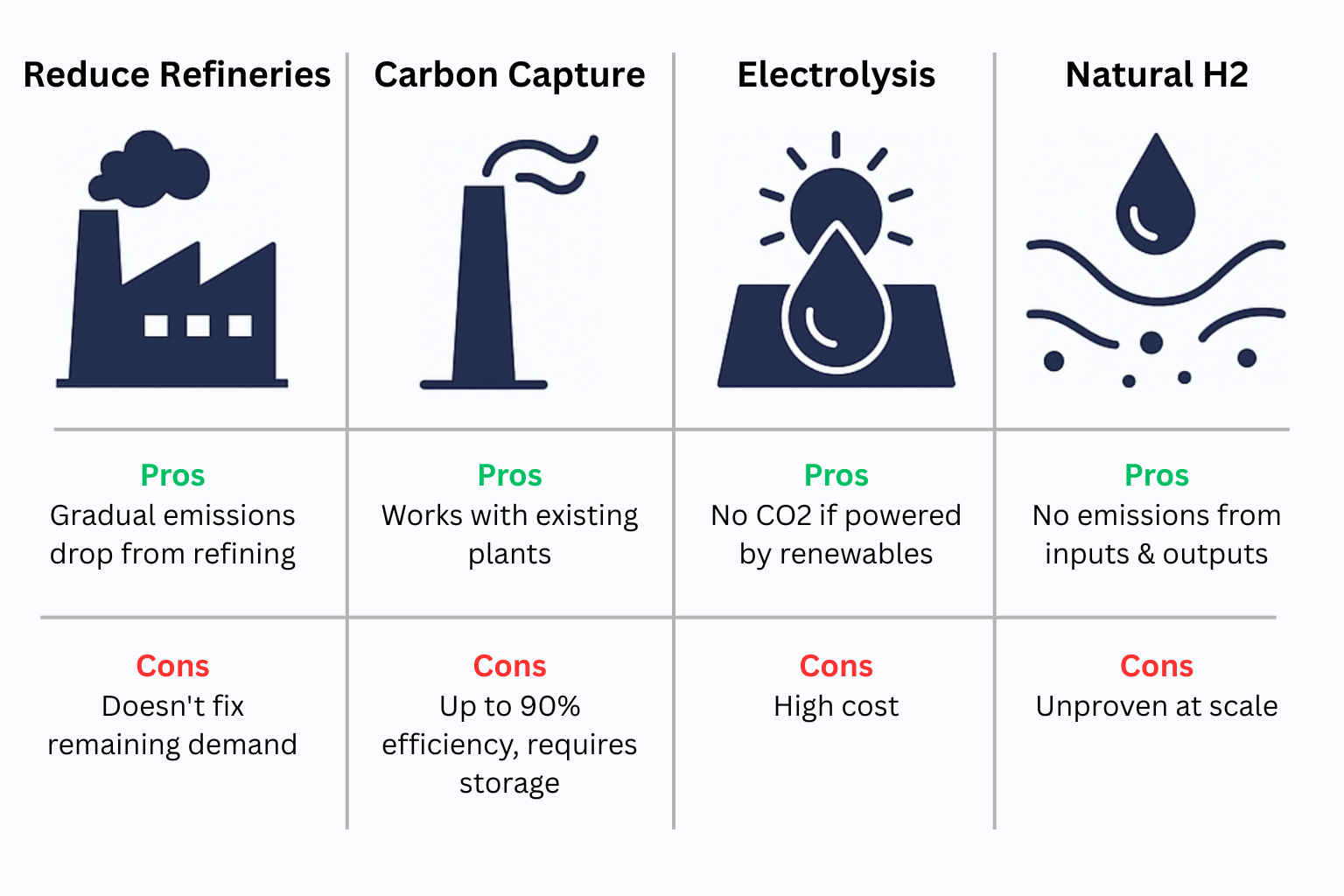
Option 1: Do Nothing - Use Less Hydrogen
As we phase out fossil fuels like diesel and petrol, hydrogen demand from refining (~43 Mt) will drop. That’s true. But even then, we’d still need around 60 million tonnes of hydrogen per year for fertilizers, methanol, steel, and other essential uses.
So simply using less is not enough to reduce hydrogen's carbon emissions.
Option 2: Carbon Capture and Storage (CCS)
We could retrofit existing hydrogen plants with carbon capture and storage (CCS) systems. These technologies capture CO₂ at the source and store it underground - often in salt caverns or depleted gas fields.
But CCS isn’t perfect:
- Capture rates rarely exceed 90%
- It requires extra energy to operate (usually fossil-based)
Global CO₂ utilization demand (e.g., for drinks, greenhouses) is only ~230 Mt/year - far less than what hydrogen alone emits.
That leaves long-term underground storage - which is technically feasible, but not yet scaled affordably worldwide.
Option 3: Electrolysis
A better solution may be electrolysis - splitting water (H₂O) into hydrogen and oxygen using electricity.
If powered by renewables, the resulting hydrogen is zero-carbon - no emissions, no fossil inputs.
This is called green hydrogen. And it’s real. But:
- Electrolyzers are expensive
- They need large volumes of cheap, clean electricity
- Infrastructure is still scaling
Still, as renewable electricity grows, electrolysis is the most scalable path to clean hydrogen production.
Option 4: Naturally Occurring Hydrogen
Finally, there’s a wild card: natural hydrogen (also called white or gold hydrogen).
Found in underground geological formations, it may be possible to extract hydrogen directly from the earth, similar to how we produce oil and gas. No CO₂, no processing, just pump and use.
It’s early-stage, but several startups and governments are exploring its potential. If proven viable, it could become a low-cost, clean hydrogen source at scale.
Conclusion: Fixing What We Already Use
Hydrogen doesn’t need to be hyped - it’s already essential. Even if future applications (hydrogen planes, long-term energy storage, fuel-cell ships) never take off, we’ll still need tens of millions of tonnes every year.
Right now, hydrogen production emits nearly a billion tonnes of CO₂ annually. That’s a climate problem we already have, not a future one.
So rather than debating whether hydrogen is a “miracle fuel” or not, let’s start by cleaning up the hydrogen we already use.
That alone would be a massive win - for energy, food, industry, and the planet.